½Chemistry + ½Biology ± Biochemistry
 This is a prelude to the Chemistry for Biology Technicians (Unit 16) and my title is meant to indicate that Biochemistry is more like the outcome of sexual reproduction in mammals rather than a simple neutralisation titration, in which you add 100ml 1MNaOH to 100ml 1MHCl! That is to say, it is an unequal partnership. Biochemistry and Molecular Biology two of the main offspring from the union of the two disciplines, are in many cases at least, two thirds Chemistry and one third Biology (ie sometimes you look more like your mum than your dad!). Which is why you always hear me droning on about the importance of Chemistry in the Life Sciences. So, why do I firmly believe this is the case? Here are 3 reasons (but there are many more) and one practical example.
This is a prelude to the Chemistry for Biology Technicians (Unit 16) and my title is meant to indicate that Biochemistry is more like the outcome of sexual reproduction in mammals rather than a simple neutralisation titration, in which you add 100ml 1MNaOH to 100ml 1MHCl! That is to say, it is an unequal partnership. Biochemistry and Molecular Biology two of the main offspring from the union of the two disciplines, are in many cases at least, two thirds Chemistry and one third Biology (ie sometimes you look more like your mum than your dad!). Which is why you always hear me droning on about the importance of Chemistry in the Life Sciences. So, why do I firmly believe this is the case? Here are 3 reasons (but there are many more) and one practical example.
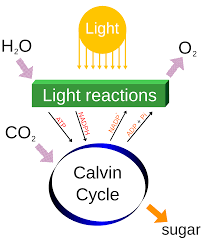
 Energy from Food. Life is a highly dynamic phenomenon (although the variously toed sloths are doing their best to disprove this generalisation (as are some individuals.....). For example as we discussed in Biochemistry, every day we turn over (ie synthesise and hydrolyse) 65kg of ATP. Yes, a whole student body mass per day! A plant transfers electrons along a set of pigments and proteins in order to convert sunlight energy into chemical energy at rates that are sub pico-seconds, whilst we can only walk (apart from Will!) at around 3 miles per hour. It is only by understanding thermodynamics and kinetics of chemical reactions that we can begin to appreciate the underlying principles of Life. Living organisms obey all of the conventional rules of Chemistry and Physics, and they use enzymes to enable them to do this at a modest temperature (in our case 37
°
C).
Energy from Food. Life is a highly dynamic phenomenon (although the variously toed sloths are doing their best to disprove this generalisation (as are some individuals.....). For example as we discussed in Biochemistry, every day we turn over (ie synthesise and hydrolyse) 65kg of ATP. Yes, a whole student body mass per day! A plant transfers electrons along a set of pigments and proteins in order to convert sunlight energy into chemical energy at rates that are sub pico-seconds, whilst we can only walk (apart from Will!) at around 3 miles per hour. It is only by understanding thermodynamics and kinetics of chemical reactions that we can begin to appreciate the underlying principles of Life. Living organisms obey all of the conventional rules of Chemistry and Physics, and they use enzymes to enable them to do this at a modest temperature (in our case 37
°
C).
 Making and breaking bonds. Our cells contain thousands of proteins, some of which are enzymes, whose job is, in the main "linking" or "dissolving" relationships between atoms. Sometimes it's the loss of a water molecule (in protein synthesis), sometimes it's the hydrolysis of a phosphate from ATP (in many metabolic reactions and protein regulation events), sometimes it's the making or breaking of carbon-carbon bonds. All of which may be facilitated by the displacement and rearrangement of electrons, or the rearrangement of a hydrogen bond here and there, or the Rubik's-cube-like twisting of hydrophobic faces, to facilitate bond formation or breakage. We must have a deep understanding of covalent, ionic, non-covalent, and hydrogen-bonds in order to design and manufacture drugs or to ensure that we avoid skin cancer by not standing in the sun or, worse still, under a sun lamp. We shall look at the pioneering work of Linus Pauling who not only provided us with the basic templates for understanding protein structure, the concepts underpinning enzymatic catalysis, but who wrote the book on the chemical bond.
Making and breaking bonds. Our cells contain thousands of proteins, some of which are enzymes, whose job is, in the main "linking" or "dissolving" relationships between atoms. Sometimes it's the loss of a water molecule (in protein synthesis), sometimes it's the hydrolysis of a phosphate from ATP (in many metabolic reactions and protein regulation events), sometimes it's the making or breaking of carbon-carbon bonds. All of which may be facilitated by the displacement and rearrangement of electrons, or the rearrangement of a hydrogen bond here and there, or the Rubik's-cube-like twisting of hydrophobic faces, to facilitate bond formation or breakage. We must have a deep understanding of covalent, ionic, non-covalent, and hydrogen-bonds in order to design and manufacture drugs or to ensure that we avoid skin cancer by not standing in the sun or, worse still, under a sun lamp. We shall look at the pioneering work of Linus Pauling who not only provided us with the basic templates for understanding protein structure, the concepts underpinning enzymatic catalysis, but who wrote the book on the chemical bond.
 Atomic structure. The shape of Biological molecules plays a major role in
defining the function of those molecules. Think of all of the Molecules of the Month that I have written about and you have read! Antibodies: three-dimensional, Y-shaped proteins that capture and eliminate foreign bodies. Think of the double helix, supremely suited for semi-conservative DNA replication. Or the beauty of Green Fluorescent Protein (RHS): a chance function in an organism that drifts around the remote oceans, that has been successfully turned into a reporter of human cell biology. We must understand the properties of atoms: their electron density(for X-ray crystallography), the quantum magnetic moment of certain nuclei (proton NMR) and the geometry that has emerged as the perfect solution to balancing all of the forces involved in atomic interactions that enables us to derive structural models to help us understand the molecular principles underpinning Life.
Atomic structure. The shape of Biological molecules plays a major role in
defining the function of those molecules. Think of all of the Molecules of the Month that I have written about and you have read! Antibodies: three-dimensional, Y-shaped proteins that capture and eliminate foreign bodies. Think of the double helix, supremely suited for semi-conservative DNA replication. Or the beauty of Green Fluorescent Protein (RHS): a chance function in an organism that drifts around the remote oceans, that has been successfully turned into a reporter of human cell biology. We must understand the properties of atoms: their electron density(for X-ray crystallography), the quantum magnetic moment of certain nuclei (proton NMR) and the geometry that has emerged as the perfect solution to balancing all of the forces involved in atomic interactions that enables us to derive structural models to help us understand the molecular principles underpinning Life.
 Acids and Bases: pH. The movement of electrons along the inner mitochondrial membrane drives the transfer of protons, which in turn cranks the rotary "engine" of ATP synthase. In this way we are able to capture the energy in our food. How do we know? We know because we can measure pH changes very precisely. When an enzyme is assayed at pH 1, 2, 3 etc, we find (in general) it prefers between pH6 and 8. Why? Because this is the pH found in most cells. The fact that we are made largely from macromolecules that can accept or lose hydrogen ions (protons) at modest pHs (between 5 and 9), means that we must understand the pH dependency of such macromolecules and their constituent building blocks. And if pH measurements are not taken accurately and solutions are not prepared correctly, all of our experiments are likely to be meaningless!
Everything above, in my view justifies the importance I place on Chemistry for anyone practising in the Life Sciences. So let's embrace Unit 16!
Acids and Bases: pH. The movement of electrons along the inner mitochondrial membrane drives the transfer of protons, which in turn cranks the rotary "engine" of ATP synthase. In this way we are able to capture the energy in our food. How do we know? We know because we can measure pH changes very precisely. When an enzyme is assayed at pH 1, 2, 3 etc, we find (in general) it prefers between pH6 and 8. Why? Because this is the pH found in most cells. The fact that we are made largely from macromolecules that can accept or lose hydrogen ions (protons) at modest pHs (between 5 and 9), means that we must understand the pH dependency of such macromolecules and their constituent building blocks. And if pH measurements are not taken accurately and solutions are not prepared correctly, all of our experiments are likely to be meaningless!
Everything above, in my view justifies the importance I place on Chemistry for anyone practising in the Life Sciences. So let's embrace Unit 16!
 Atomic structure. The shape of Biological molecules plays a major role in
Atomic structure. The shape of Biological molecules plays a major role in
No comments:
Post a Comment